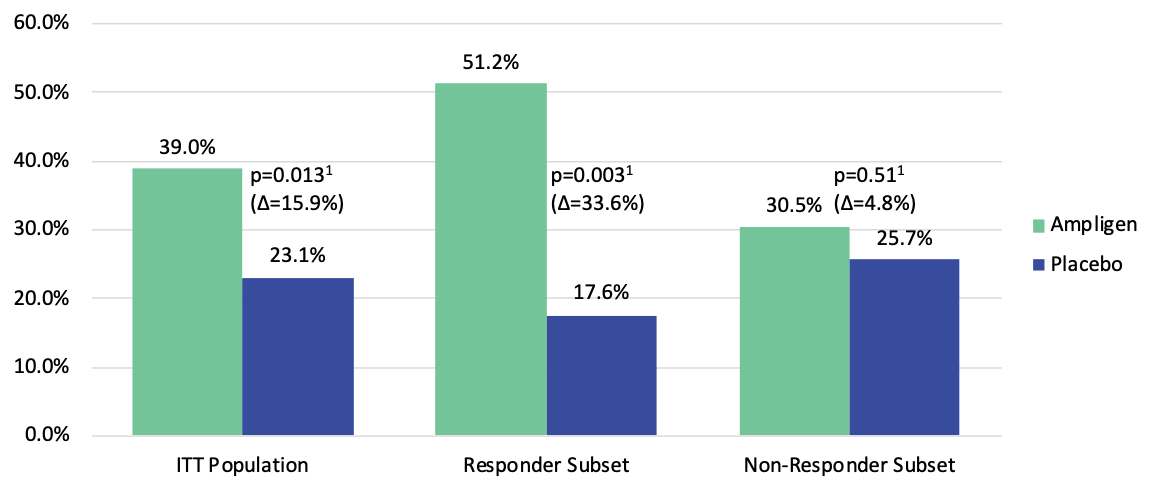Approved for the treatment of Severe CFS in Argentina
Only late-stage program in development in the U.S. for the treatment of ME/CFS
Developing protocol for confirmatory Phase 3 trial, required for U.S. NDA
ME/CFS is a disease characterized by profound fatigue, sleep abnormalities and pain. ME/CFS occurs more commonly in women and the cause of the condition is unknown. It is estimated that approximately 2.5 million people in the U.S. suffer from ME/CFS.1
Positive Results from Phase 3 Study Showed Significant Improvement in the Primary Endpoint, Exercise Treadmill Tolerance (ETT)
Strayer DR, Young D, Mitchell WM (2020) Effect of disease duration in a randomized Phase III trial of rintatolimod, an immune modulator for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. PLOS ONE 15(10): e0240403. https://doi.org/10.1371/journal.pone.0240403