Goal is to unleash the cellular immune response to attack and destroy cancer cells and increase survival1
Ampligen® is the only known TLR3 agonist to promote selective attraction of CTLs (Teff) with concomitant reduction in Treg attraction in the tumor microenvironment1
Ampligen® has been shown to suppress tumor cell proliferation in those cancer cells expressing TLR32
- Muthuswamy, et al. 2012 2. Theodoraki, et al. 2018
Targeting Multiple Cancers with High-Unmet Need
- National Cancer Institute: Surveillance, Epidemiology and End Results – Ovarian Cancer; Pancreatic Cancer; Breast Cancer; Melanoma;
- NIH National Cancer Institute Surgery for Recurrent Ovarian Cancer Does Not Improve Survival, December 10, 2019, by NCI Staff
- Manjunath M, Choudhary B. Triple-negative breast cancer: A run-through of features, classification and current therapies. Oncol Lett. 2021;22(1):512. doi:10.3892/ol.2021.12773
Compelling Data Generated to Date
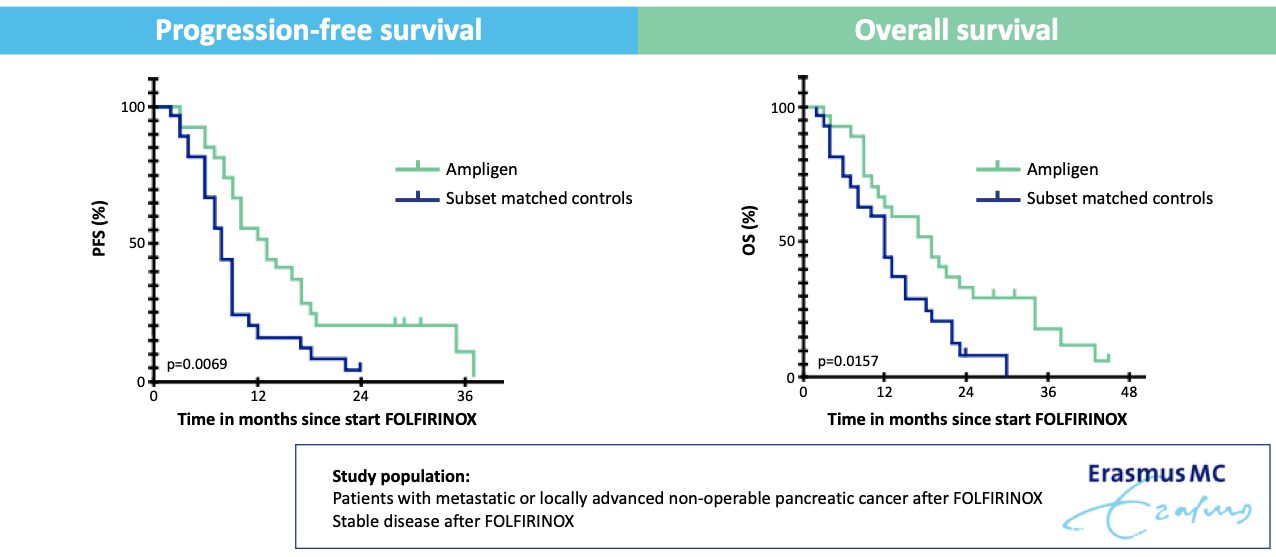
Ampligen® Maintenance Therapy after FOLFIRINOX Improves Overall and Progression-Free Survival in Pancreatic Cancer
el Haddaoui H, Brood R, Latifi D, Oostvogels AA, Klaver Y, Moskie M, Mustafa DA, Debets R, van Eijck CHJ. Rintatolimod (Ampligen®) Enhances Numbers of Peripheral B Cells and Is Associated with Longer Survival in Patients with Locally Advanced and Metastasized Pancreatic Cancer Pre-Treated with FOLFIRINOX: A Single-Center Named Patient Program. Cancers. 2022; 14(6):1377. https://doi.org/10.3390/cancers14061377
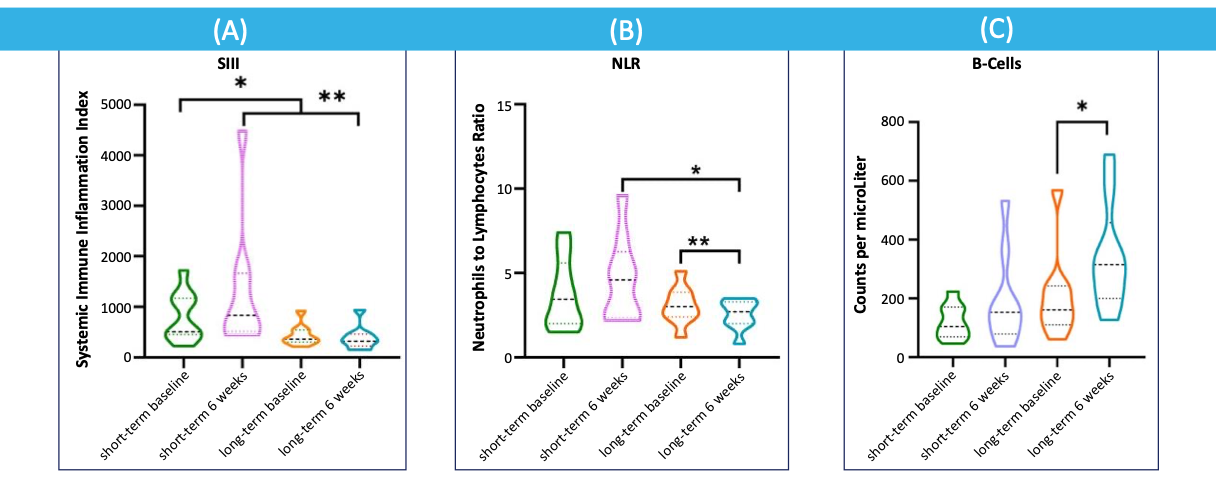
Pancreatic Cancer: Long-Term Survivors Exhibit Decreased SIII and NLR as well as Increased B-Cell Counts
A.Systemic immune inflammation Index (SIII) in short-term survivors (n=9) and long-term survivors (n=9). * p=0.010, ** p=0.005 (Mann-Whitney U test).
B.Neutrophil to lymphocyte ratio (NLR) in short-term and long-term survivors * p=0.006, ** p=0.014 (Mann-Whitney U test).
C.B cells in circulating blood of short-term and long-term survivors * p=0.002 (Mann-Whitney U test). el Haddaoui Cancers 2022, 14, 1377. https://doi.org/10.3390/cancers14061377
About Ampligen®
Ampligen is currently being used to treat pancreatic cancer patients in an Early Access Program (EAP) approved by the Inspectorate of Healthcare in the Netherlands at Erasmus Medical Center and AIM has commenced a Phase 2 clinical study in locally advanced pancreatic cancer. The Company also has multiple ongoing clinical trials to evaluate Ampligen as a combinational therapy for the treatment of a variety of solid tumor types both underway and planned at major cancer research centers. Additionally, Ampligen is approved in Argentina for the treatment of severe chronic fatigue syndrome (CFS) and is currently being evaluated in many aspects of SARS-CoV-2/COVID-19 myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and post-COVID conditions.

![Silo Background_1011906997 [Converted]-01](https://aimimmuno.com/wp-content/uploads/2023/03/Silo-Background_1011906997-Converted-01.png)